views
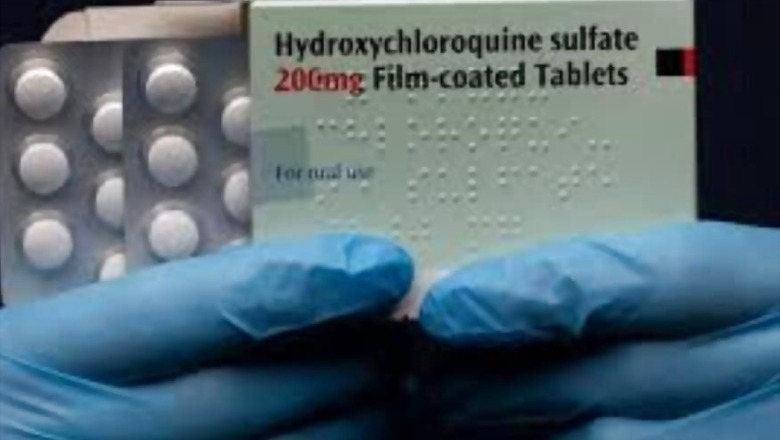
Washington: A consignment of hydroxychloroquine from India arrived in the United States on Saturday, days after New Delhi lifted a ban on export of the anti-malaria drug, seen as a possible cure for COVID-19, to the US and some other countries on humanitarian grounds.
Earlier this week, India at the request of President Donald Trump cleared the export of 35.82 lakh tablets of hydroxychloroquine to the US along with nine metric tons of active pharmaceutical ingredient or API required in the manufacturing of the drug.
"Supporting our partners in the fight against COVID-19. Consignment of hydroxychloroquine from India arrived at Newark airport today," India's Ambassador to the US Taranjit Singh Sandhu tweeted.
Trump, during a phone call last week, asked Prime Minister Narendra Modi to lift the hold on American order of the anti-malarial drug, of which India is the major producer. India, which manufactures 70 per cent of the world's supply of hydroxychloroquine, lifted the ban on April 7.
Hydroxychloroquine has been identified by the US Food and Drug Administration as a possible treatment for the COVID-19 and it is being tested on more than 1,500 coronavirus patients in New York.
Anticipating that it will work, given initial positive results, Trump has bought more than 29 million doses of hydroxychloroquine for potential treatment of COVID-19 patients.
The arrival of consignment was welcomed by Americans. "US will never forget this great humanitarian gesture by India. Under President Trump and Prime Minister Narendra Modi, the two largest democracies of the world have come together than ever in the past, said New York-based Al Mason, a real estate consultant and a Trump supporter.
A wonderful gesture by India to its friends in need, tweeted Dr Sampat Shivangi. Describing this as a wonderful gesture, Steve Filipovic, who works in the construction industry, thanked the Indian Ambassador.
Meanwhile, according to a new French study of 1,061 hospitalised COVID-19 patients showed a 91.7 per cent cure (viral shedding) within 10 days of hydroxychloroquine treatment along with anti-biotic. It also reported a 96 per cent cure rate after 15 days.
Early this week, the National Institute of Health reported that a clinical trial to evaluate the safety and effectiveness of hydroxychloroquine for the treatment of adults hospitalised with the coronavirus disease 2019 (COVID-19) has begun, with the first participants now enrolled in Tennessee.
Hydroxychloroquine is used to treat malaria and rheumatoid conditions such as arthritis. In various studies, the drug has demonstrated antiviral activity, an ability to modify the activity of the immune system, and has an established safety profile at appropriate doses, leading to the hypothesis that it may also be useful in the treatment of COVID-19, NIH said.
The drug is not without risks as even short-term use can cause cardiac arrythmias, seizures, dermatological reactions, and hypoglycemia, it added.
Many US hospitals are currently using hydroxychloroquine as first-line therapy for hospitalized patients with COVID-19 despite extremely limited clinical data supporting its effectiveness,� said Wesley Self, emergency medicine physician at Vanderbilt University Medical Center and PETAL Clinical Trials Network investigator leading the ORCHID trial.
Thus, data on hydroxychloroquine for the treatment of COVID-19 are urgently needed to inform clinical practice, he added.
According to NIH, participants will be randomly assigned to receive hydroxychloroquine 400 mg twice daily for two doses (day one), then 200 mg twice daily for the subsequent eight doses (days two to five) or a placebo twice daily for five days.
COVID-19 cases were first identified in December 2019 in Wuhan, Hubei Province, China.
As of Saturday, more than 16 lakh people globally tested positive and more than one lakh have died. The United States has emerged as the hotspot with 20,000 deaths and 5.3 lakh cases.



















Comments
0 comment