views
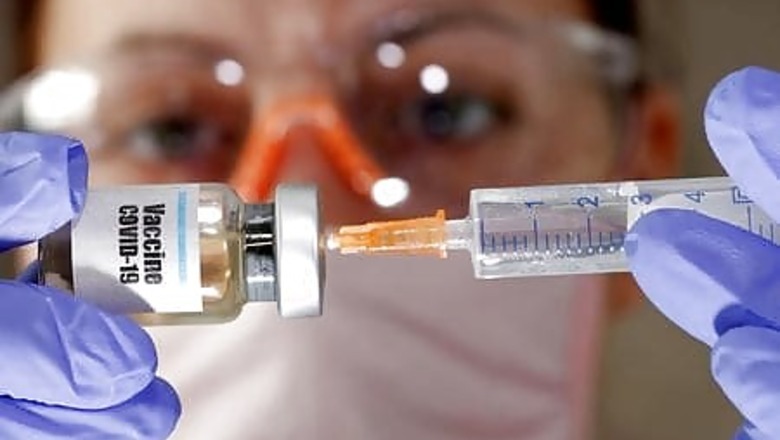
The U.S. Food and Drug Administration will organize meetings with an independent group of experts to review data of coronavirus vaccine candidates and advise the agency, FDA commissioner Stephen Hahn said on Wednesday.
“The meetings will reinforce the transparency of the process as FDA reviews data from trials now underway,” Hahn said in a post on Twitter. (https://bit.ly/2YYBHI0)
The statement comes after U.S. President Donald Trump last month accused members, without evidence, of a so-called “deep state” working within the FDA of complicating efforts to test COVID-19 vaccines in order to delay results until after the Nov. 3 presidential election.
The FDA said last week it will hold a meeting of its advisory committee to address the general development of COVID-19 vaccines on Oct. 22, and have additional meetings as applications for coronavirus vaccines are submitted.
The agency typically follows the advice of its experts, although it is not bound to do so.
While several drugmakers including AstraZeneca Plc and Moderna Inc are conducting late-stage studies of their experimental coronavirus vaccines, there are no U.S.-approved vaccines for the novel coronavirus.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor


















Comments
0 comment