views
Inspecting the Surface
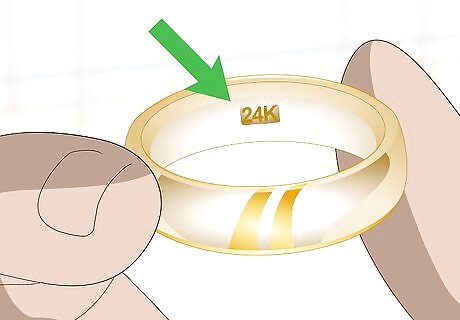
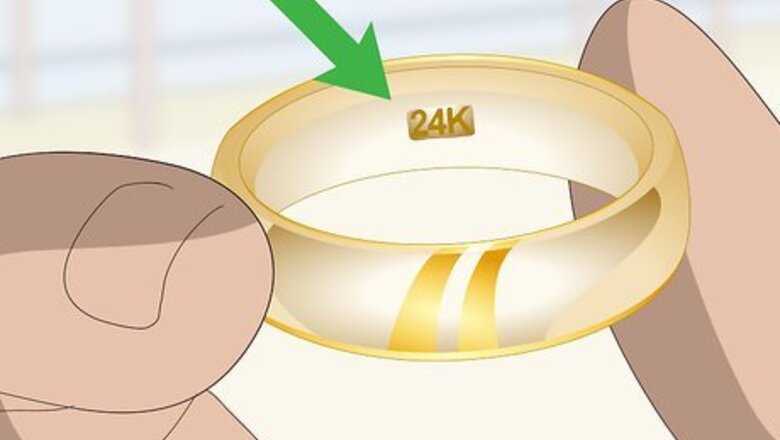
Look for a hallmark. A piece of gold will usually be stamped with a mark indicating its type. A stamp of “GF” or “HGP” indicates that the piece is gold-plated, not pure gold. In contrast, a pure gold piece of jewelry may show a “24K” or other marking indicating fineness. Hallmarks are usually located inside the band of rings or near the clasp on necklaces. However, be aware that some hallmarks can be faked. This is why it’s important to use a mark as only one of many indicators of authenticity. The hallmark may be very small. You might even need a magnifying glass in order to see it clearly.
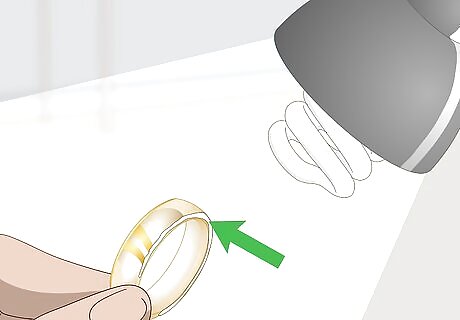
Look for fading around the edges of the piece. Turn on a bright light or lamp. Hold the piece close to the lamp’s light. Rotate it in your hand, so that you can examine all of the edges in particular. If you see that the gold appears faded or worn away at the edges, then it’s likely wear on the plating. This means that piece isn’t pure gold.
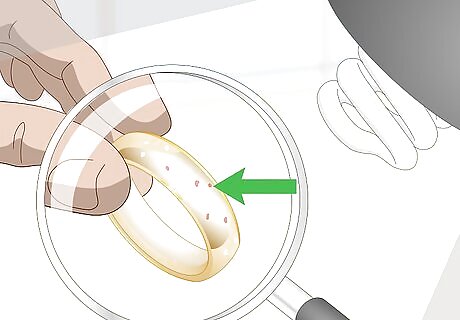
Look for spotting on the piece’s surface. If you hold the piece under a bright light, do you notice white or red spots anywhere on it? The spots may be very tiny and difficult to see. That is why it’s important to examine the piece under a bright light and maybe with a magnifying glass. These spots indicate that the gold plating may be wearing away showing the metal underneath.
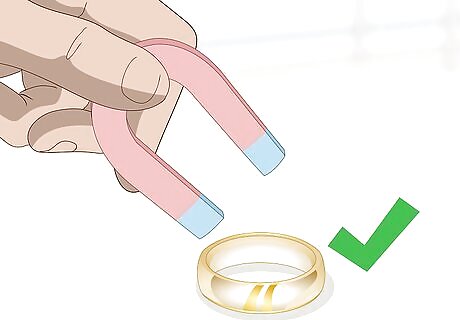
Place a magnet against the potential gold item. Hold a magnet directly above the piece. Lower the magnet until it is almost touches the surface of the item. If you feel as if the magnet is being drawn or pulled downward, then the item is not pure. The other metals in the item, such as nickel, are responding to the magnet. A pure gold piece will not draw the magnet, since non-ferrous.
Conducting Deeper Testing
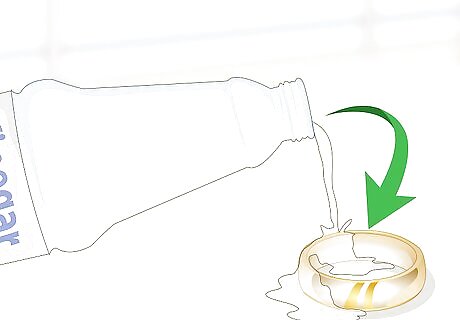
Apply some vinegar to the surface and look for a color change. Get a dropper and fill it up with white vinegar. Hold your metal object firmly in your hand or set it on a table. Place a few drops of vinegar onto the object. If the drops change the color of the metal, then it is not pure gold. If the color stays the same, then it is pure gold.
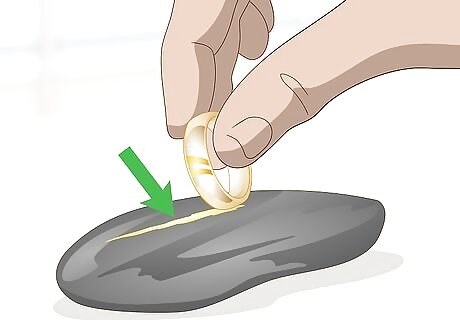
Rub your gold against a jeweler’s stone. Position a black jeweler’s stone on a table. Hold your gold piece firmly in your hand. Wipe it across the stone firmly enough to leave a mark. If the mark that you’ve left on the stone is solid and gold in color, then the piece is pure. If there is no line or only a faint one, then the piece is likely plated or not gold at all. Be careful with this method as you run the risk of damaging your jewelry. You also have to use the right type of stone or the marks will be meaningless. You can get a jeweler’s stone through a jewelry supply store online or by talking with your local jeweler.
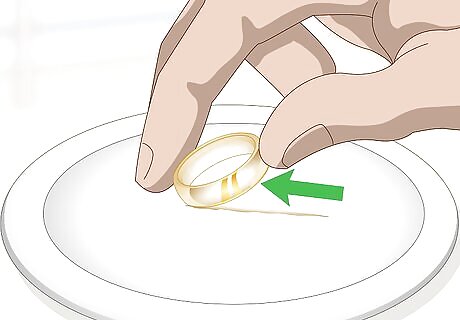
Rub your gold across a ceramic plate. Set an unglazed ceramic plate firmly on a countertop or table. Hold your gold item in your hand. Scrape the item against the plate. Watch to see if a streak or line of any type appears. A black line indicates that the item is not gold or is plated.
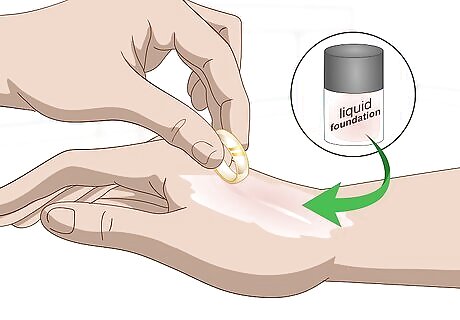
Test your gold against liquid foundation makeup. Coat the top of your hand with a thin layer of liquid foundation. Wait until the foundation is dry. Press your metal item against the foundation and rub. Authentic pure gold will leave a line in the makeup. If you do not see a line, then the object is plated or another metal.

Use an electronic gold tester. This is a small hand-held device with a probe at the end that you can buy online or through a jewelry supply store. To analyze a metal, you rub a conductive “tester” gel on to the metal item. This gel is usually available for purchase from the same places that sell testing devices. After you’ve applied the gel, rub the probe against the item. How the metal responds to the electricity will indicate whether or not it is pure metal. Use the instructions that come with your tester to determine the exact results. Gold is a conductive metal, so a pure gold piece will have higher readings than a plated one.
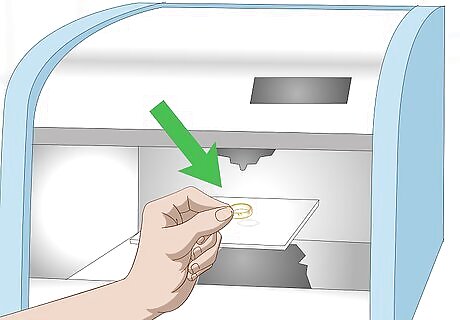
Insert your gold into an XRF machine. This is a machine that many jewelers use to instantly determine the quality of a metal sample. Because of its cost this method may not be suitable for home use, unless you plan on using it regularly. To use an XRF scanner, place the piece of metal inside, activate the machine, and wait for the read out.
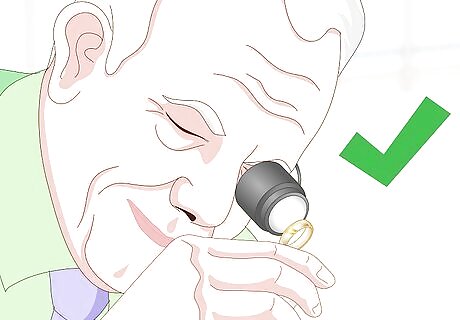
Take your gold to an assayer. If you keep getting mixed results or if you’d like to verify your finding, talk to your jeweler about getting another professional opinion. An assayer will perform a deep analysis of the content of the metal. This can be a costly option, so only use it if you believe your item may be worthwhile.
Performing Acid Testing
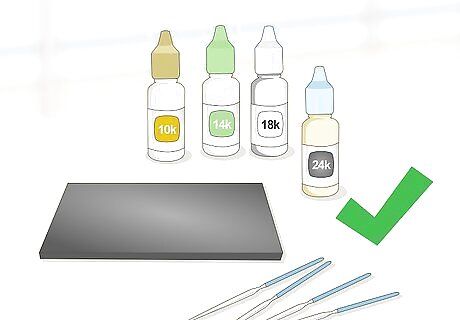
Buy an acid testing kit for a more precise estimate of gold karat purity. You can purchase one of these kits through a jewelry tool supplier. The kit will contain all of the materials that you’ll need along with a set of detailed instructions. Make sure to read the instructions carefully before beginning and conduct an inventory of the supplies before starting. These kits can be quite affordable, if ordered online. They start at around $30.
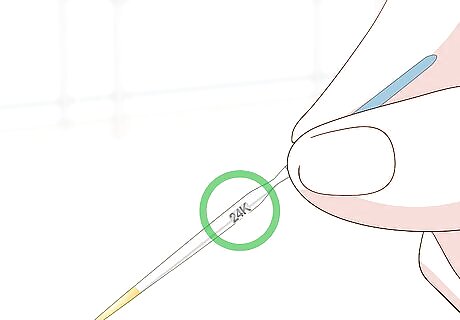
Inspect the needles for karat value labels. Your kit will contain a number of needles that you’ll use for testing different types of gold. Look for a karat value marking on the side of the needle. Each needle will also have a colored gold sample at the tip. Use the yellow needle for yellow gold and the white needle for white gold.
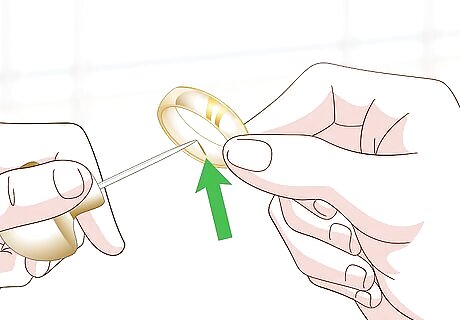
Make a notch with an engraving tool. Turn the piece around until you find a less noticeable spot. Hold an engraving tool firmly in your hand and make a small divot in the metal. The goal is to expose the deeper layers of the metal.
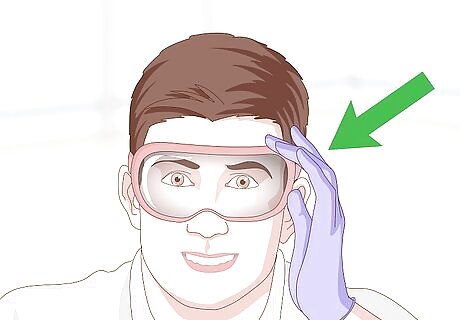
Put on protective gloves and goggles. Since you are working with acid, it’s important to don thick, but fitted, gloves. Eye protection is also a good idea, just to be extra cautious. Avoid touching your face or your eyes while working with the acid.
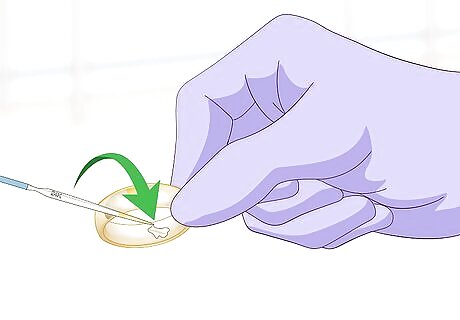
Place a drop of acid on the notch. Select the proper needle for the gold type. Then, hold the needle tip directly over the notch. Push the plunger of the needle down until a single drop of acid drops into the divot.
Read the results. Look closely at the divot that you made earlier and where you just applied the acid. The acid will react with the metal and may turn a particular color. Generally, if the acid turns a green color, this indicates that the piece is not pure metal, but instead gold plated or another metal entirely. Since testing kits have different color indications, make sure to read the color guide carefully as you interpret the test results. You can also do the nitric acid test. Nitric acid can help determine the presence of base metals in gold items. Apply a small amount of nitric acid to a discreet part of the item. Observe any color changes; if the acid causes the metal to turn green, it may indicate a lower gold content.















Comments
0 comment