views
New Delhi: News of the early success of vaccine candidates -- from Israel and Italy -- on Wednesday made it to headlines across the globe. The defence ministry of Israel announced a breakthrough in the development of an antibody while biotech firm Takis in Italy said vaccine tests conducted at Lazzaro Spallanzani National Institute for Infectious Diseases in Rome had shown positive effects.
So what are these vaccines and what effects have they shown? News18 explains.
What are the claims made by Israel’s defence ministry about its vaccine?
Israel's Defence Minister Naftali Bennett said scientists at the Israel Institute of Biological Research (IIBR) had developed an antibody to COVID-19. Bennett claimed it was a "significant breakthrough" and that researchers are moving to patent and mass-produce the potential treatment.
The IIBR is a secretive organisation that works directly under the Israel Prime Minister’s Office. The Times of Israel reported that the antibody’s development had been completed and that researchers will approach international companies to produce the antibody on a commercial scale. However, the report also said that the antibodies were yet to be tested on humans.
What are the claims made by the Italian biotech firm?
Takis in collaboration with Applied DNA Sciences Inc. is working on a vaccine. The company was carrying out animal studies at the Lazzaro Spallanzani National Institute for Infectious Diseases in Rome. It said on Wednesday that during animal studies their vaccine produced antibodies that had a neutralising effect on the virus, according to the Associated Press. Dr Luigi Aurisicchio, CEO and chief scientific officer at Takis, reportedly said: “Thanks to the Spallanzani’s kills, as far as we know, we are among the first in the world to have demonstrated the neutralisation of the coronavirus by a vaccine. We believe this will also happen in humans.”
According to reports, the company is likely to commence human trials soon. The DNA-based vaccine was able to produce an antibody response against the spoke protein of the virus in mice.
What is the WHO’s solidarity trial?
To integrate some of the overlapping efforts of scientists from across the world to find an effective treatment for the novel coronavirus, the World Health Organization (WHO) launched an international ‘solidarity clinical trial’. This will compare four treatment options against the standard of care to assess their relative effectiveness against COVID-19, it said. One of the crucial reasons behind this effort is to expedite the process of trials and increase its scale.
It usually takes years to design and conduct clinical trials for emerging diseases. WHO said the solidarity trial will reduce this by 80%.
“Enrolling patients in one single randomised trial will help facilitate the rapid worldwide comparison of unproven treatments. This will overcome the risk of multiple small trials not generating the strong evidence needed to determine the relative effectiveness of potential treatments,” it said. Over 100 countries are working together on this issue.
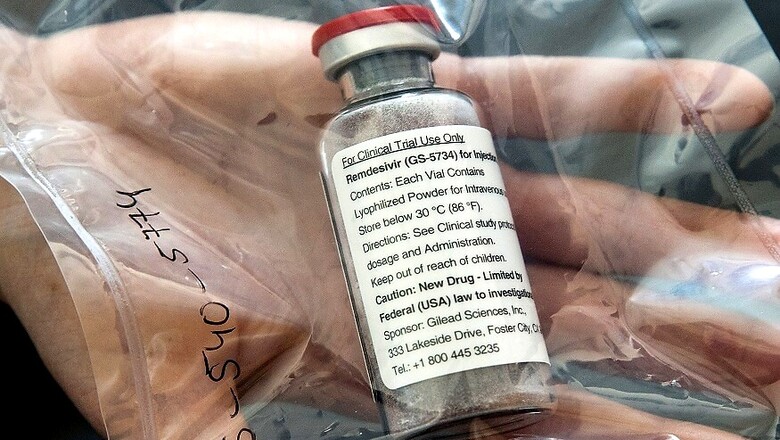
Based on evidence from laboratory, animal and clinical studies, Remdesivir, Lopinavir/Ritonavir, Lopinavir/Ritonavir with Interferon beta-1a and Chloroquine, or Hydroxychloroquine were the options selected for the trials.

















Comments
0 comment