185
views
views
Have you ever had to look up a value in a table only to find that the conditions you have are found in between those that are listed? What did you do when this happened? You probably just rounded off. An alternative way is to interpolate. This is a more accurate way of getting the desired value proportionally from a table when the conditions are not listed (see the "Warning" section below).
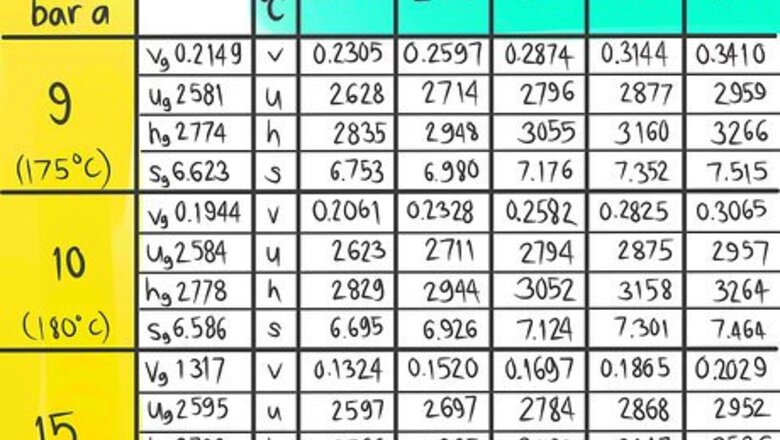
A steam table (which lists Temperature and Pressure conditions to give Enthalpy, Entropy, Specific Volume and Specific Internal Energy values) is an example of a table that may need interpolation. The following instructions will teach you how to do a double linear interpolation. For this demonstration, use the steam table to find the Enthalpy (h) at the conditions 12 bar a, which is designated as A, and 325 C, which is called B in this article.
enthalpy the measure of the heat content as a relation in a system as in chemical analysis by heating and then recording the temperature of a phase change, for example: when it changes from a solid to liquid or to a gas to help identify a substance.
A steam table (which lists Temperature and Pressure conditions to give Enthalpy, Entropy, Specific Volume and Specific Internal Energy values) is an example of a table that may need interpolation. The following instructions will teach you how to do a double linear interpolation. For this demonstration, use the steam table to find the Enthalpy (h) at the conditions 12 bar a, which is designated as A, and 325 C, which is called B in this article.
enthalpy the measure of the heat content as a relation in a system as in chemical analysis by heating and then recording the temperature of a phase change, for example: when it changes from a solid to liquid or to a gas to help identify a substance.
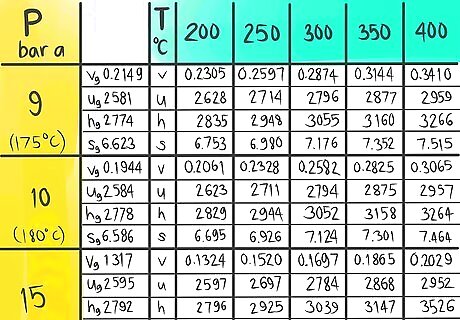
Click on the image of the example steam table to open that image in a new window in a larger size to be easier to see it clearly.
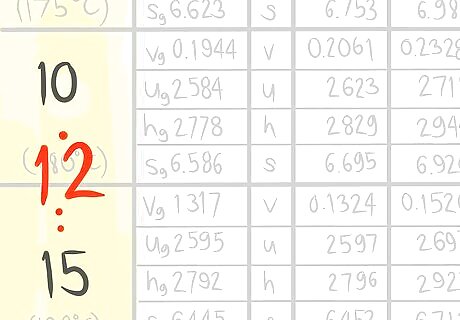
Locate where 12 bar a (A) would be.
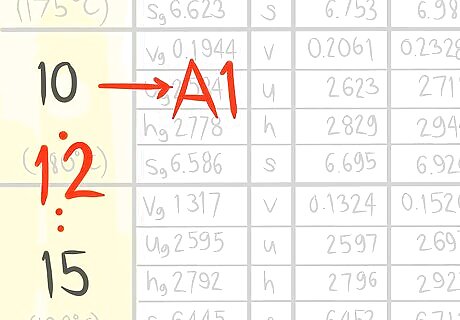
Call the value that comes before A1.
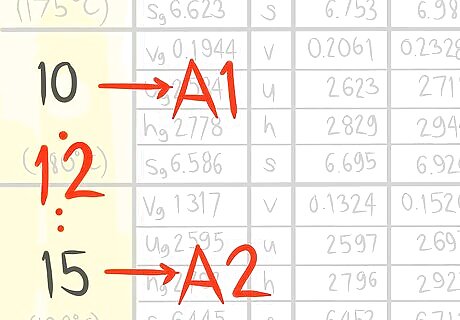
Call the value that comes after A2.
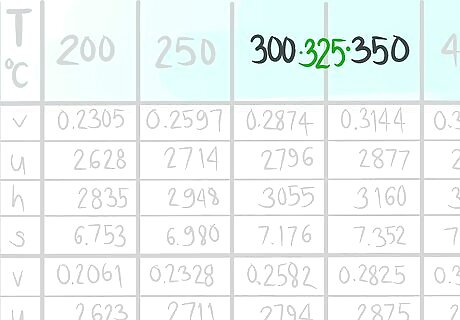
Locate where 325 C (B) would be.
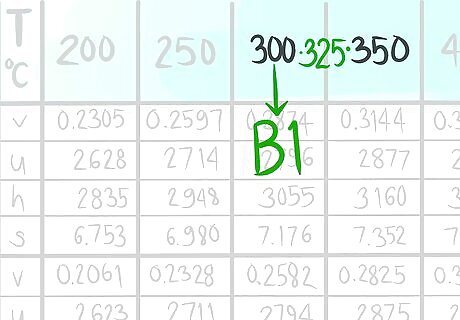
Call the value that comes before B1.
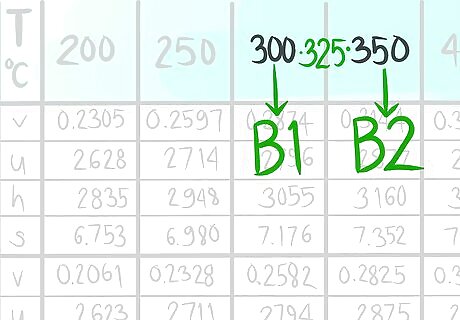
Call the value that comes after B2.
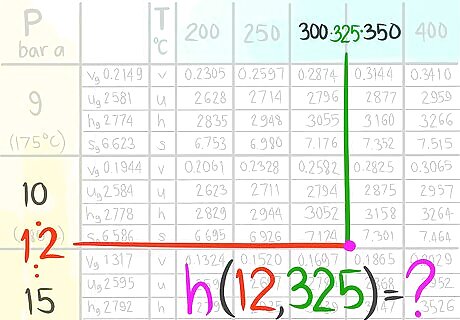
Locate where the enthalpy value would be at 12 bar a and 325 C.
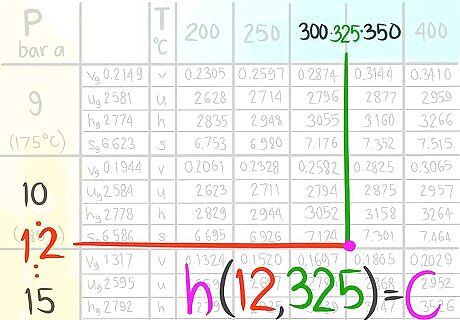
Call this enthalpy value C.
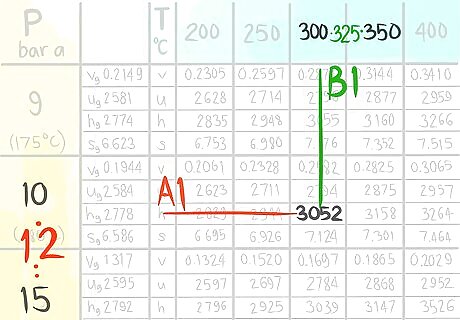
Locate the value at (A1,B1).
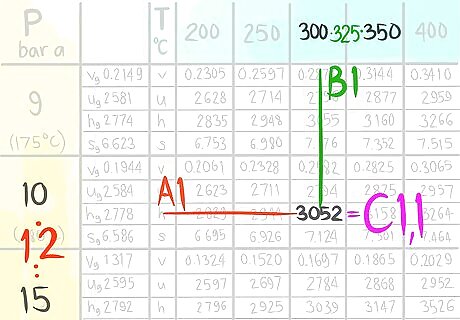
Call the value C1,1.
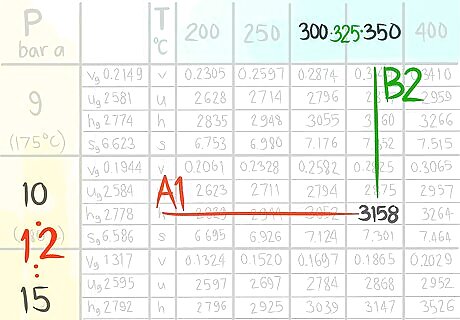
Locate the value at (A1,B2).
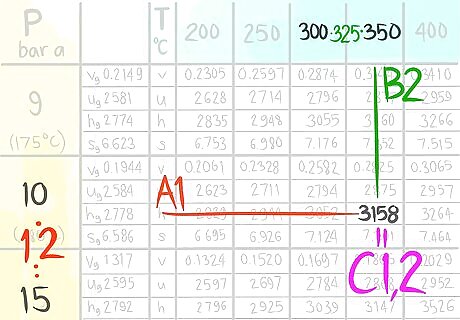
Call the value C1,2.
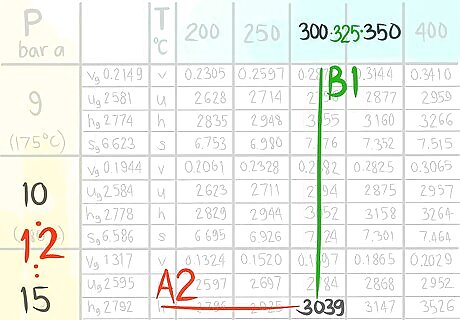
Locate the value at (A2,B1).
Call the value C2,1.
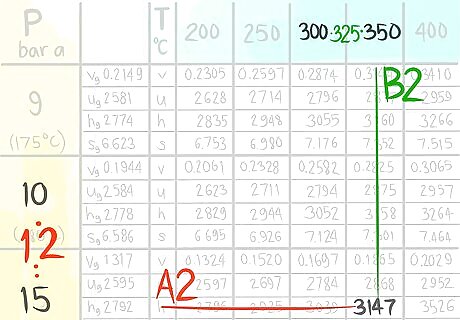
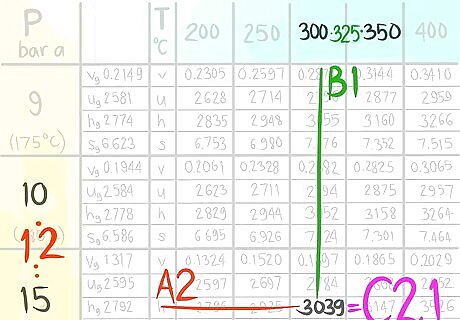
Locate the value at (A2,B2).
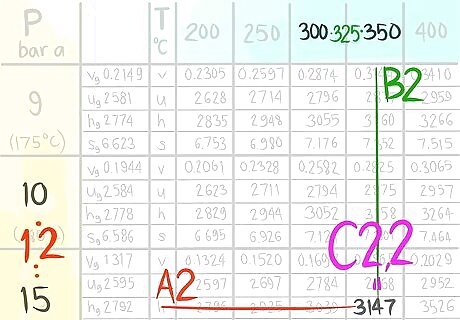
Call the value C2,2.
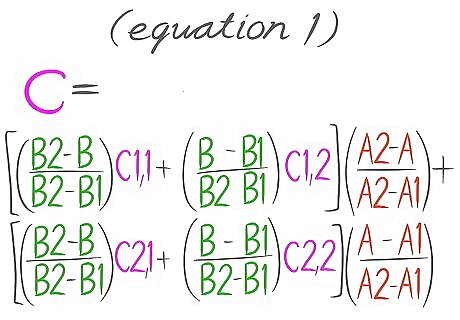
This is the Equation 1.
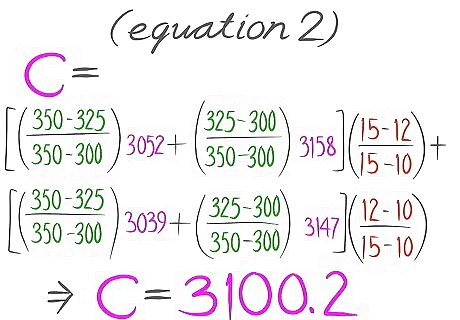
Plug values in Equation 1. It should then look like Equation 2 with values in place of the variables.
















Comments
0 comment