views
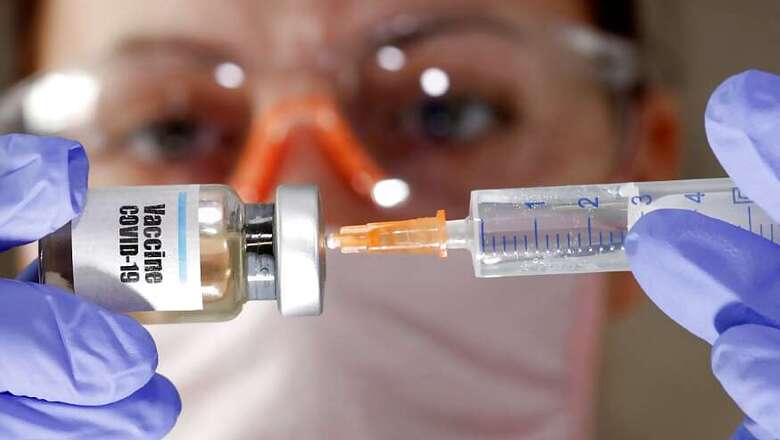
Scientists at the Oxford University said their experimental coronavirus vaccine, being developed with UK’s AstraZeneca, has shown in an early trial that it can prompt a protective immune response in hundreds of people who got the shot. With this announcement, there finally appears to be light at the end of the tunnel.
In research published on Monday in the medical journal Lancet, scientists said that they found their experimental COVID-19 vaccine produced a dual immune response in people aged between 15 to 55 who were administered the dose.
"Now what this vaccine does particularly well is trigger both arms of the immune system in addition to neutralizing antibodies which other vaccines do, we also see a very strong T-cell response," says Professor Adrian Hill, Director of the Jenner Institute, Oxford University.
The phase 1 and phase 2 results of the vaccine, called AZD1222, have been published even as the vaccine requires larger trials to test whether it can offer protection against coronavirus. The third phase is currently underway. Britain has already secured a supply of 100 million doses of the vaccine, which is being developed at an unprecedented speed.
Phase 2 and phase 3 trials for the Oxford project kicked off in May.
But what about India?
India will be procuring this vaccine from the Serum Institute of India which has a manufacturing deal with AstraZeneca to manufacture doses of this vaccine for India and other low-income countries. India's Serum Institute, the largest vaccine manufacturer in the world, has sought the DCGI’s permission to begin human trials of the vaccine.
In a statement, CEO of Serum Institute of India said, "The trials have shown promising results and we are extremely happy about it. We will be applying for the licensure trials to the Indian regulator in a week's time. As soon as they grant us permission, we will begin with the trials for the vaccine in India. In addition, we will soon start manufacturing the vaccine in large volumes, the institute will manufacture and supply one billion doses of the vaccine."
The Serum Institute of India is planning to begin human trials for the AstraZeneca Oxford Vaccine from August. However, concerns about greater access remain.
"Even two billion doses may not be enough, that's a huge achievement if we can do it. No vaccine has ever produced half a billion doses in a year. So, we definitely want other vaccines to work - we want them to share the activity, or the burden, if you like," says Professor Adrian Hill, Director of the Jenner Institute, Oxford University.
The WHO too maintained that access is a bigger challenge. "The challenge is going to be when vaccines do prove clinically efficacious, ensuring there's enough production to supply people around the world," says Dr Mike Ryan, Executive Director of WHO Health Emergencies Programme.


















Comments
0 comment