views
In the wake of the coronavirus pandemic, scientists and pharmaceutical companies across the world are hard at work to develop a vaccine. More than 140 experimental drug treatments and vaccines for COVID-19 are currently being developed and 11 of these are already in various stages of clinical trials, a report by The Wall Street Journal stated.
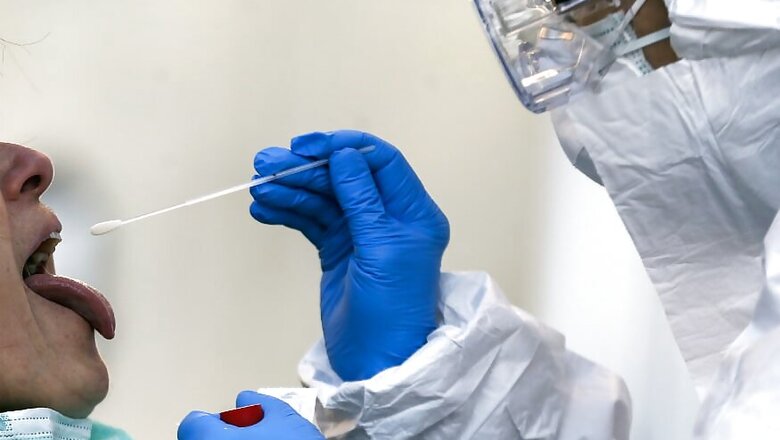
In India, Hyderabad-based vaccine manufacturer Bharat Biotech, which has been working on developing an intra-nasal drop vaccine for COVID-19, has initiated testing in the United States in collaboration with the University of Wisconsin-Madison and vaccine developer FluGen, CNBC-TV18 reported on Tuesday.
The development comes a week after Pune-based Serum Institute said it hopes to begin human trials for its vaccine by the end of the year.
Meanwhile, Bharat Biotech is hoping that its CoroFlu vaccine is ready for human trials by the end of this year.
“We are working with one of the COVID-19 nasal-flu vaccines in collaboration with University of Wisconsin. We have completed R&D experiments and now it is going through animal trials," Dr Krishna Ella, Chairman and MD of Bharat Biotech, told CNBC-TV18. "We are looking at animals because as the vaccine has some problems similar to that of dengue. So, you need to be cautious in animal testing to ensure the safety of people."
Ella said the company chose a nasal strategy since the flu and COVID-19 or any other respiratory pathogen enters through the nose and gets into the lungs.
"So, it it best to enhance mucosal immunity, which is one of the best immunity the human body can produce against any infectious disease," he said. "Hence, the nasal dose is the best option for mucosal immunity and that will last longer and protect against any infections.”
Speaking about bottlenecks in terms of research and development in India, Ella said, “Animal testing will take another two-three months to check if everything is alright, the immunogenosity is alright and the efficacy – it protects against the challenge of the virus. We need to establish the above thee points and that is happening at the moment at Madison-Wisconsin. The reason we have to do this abroad is because we do not have animals in the country [India]. If we want to import animals, then it takes about a year. So, I do not think we can do a lot of R&D in the country, that is one of the bottlenecks we face as Indian manufacturers.”
Ella said the vaccine platform they are working on has already been proven in phase-two human trials.
“FluGen has completed using the vaccine. We are already working on cell culture, vector and have established the safety profile of the product. We have easier ways to fast track a lot of things. We expect that in the next four months human clinical trials would begin,” he said.
“We have already established the dose required for humans. This gives a lot of confidence to move forward faster,” he added.


















Comments
0 comment