views
Drawing Diatomic Covalent Structures
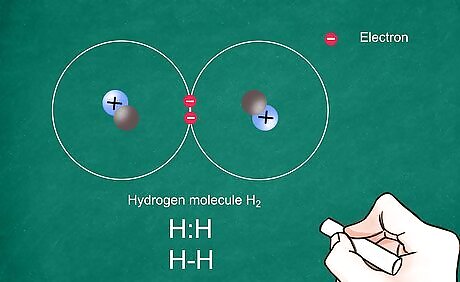
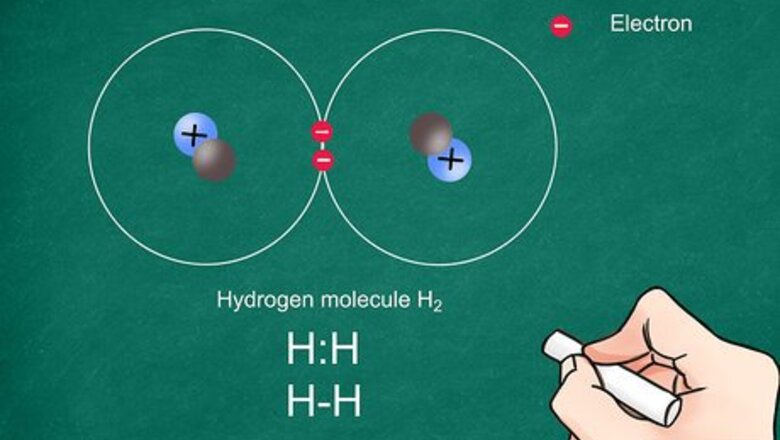
Write the atomic symbol for each atom. Write the 2 atomic symbols side by side. These symbols will represent the atoms present in the covalent bond. Be sure to leave enough space between the atoms to draw your electrons and bonds. Covalent bonds share electrons and generally occur between 2 nonmetals.
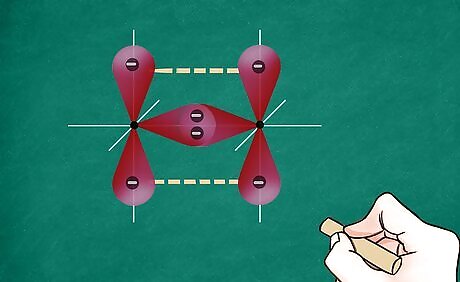
Determine the degree of the bond between the 2 atoms. Atoms can be held together by a single, double, or triple bond. Generally, this will be dictated by the octet rule, or each atom’s desire to reach a full valence shell with 8 electrons (or in the case of hydrogen, 2 electrons). To determine how many electrons each atom will have, find out how many valence electrons are in the molecule, multiply that by 2 (each bond involves 2 electrons), and then add the number of unshared electrons. For instance, O2 (oxygen gas) has 6 valence electrons. Multiply 6 by 2, which equals 12. To determine if the octet rule has been met, use dots to represent the valence electrons around each atom. For O2, one oxygen has 8 electrons (so the octet rule has been met), but the other has only 6 (so the octet rule has not been met). This signifies that more than 1 bond is required between the 2 oxygens. Therefore, 2 of the electrons are required to make a double bond between the atoms so the octet rule is met for both.
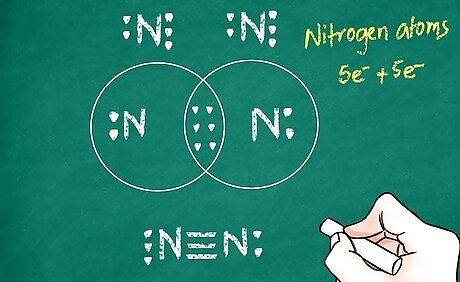
Add your bonds to the drawing. Each bond is represented with a line between the 2 atoms. For a single bond, you would simply draw 1 line from the first atom to the second. For a double or triple bond, you would draw 2 or 3 lines respectively. For example, N2 (nitrogen gas) has a triple bond connecting the 2 nitrogen atoms. So, its bond will be notated in a Lewis diagram as 3 parallel lines connecting the 2 N atoms.
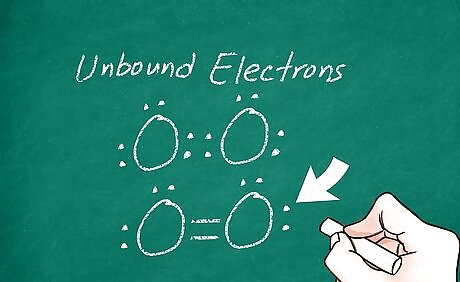
Draw unbound electrons. Some of the valence electrons in one or both of the atoms may not be involved in a bond. When this happens, you should represent each remaining electron with a dot around its respective atom. In most cases, neither atom should have more than 8 electrons bound to it. You can check your work by counting each dot as 1 electron and each line as 2 electrons. For example, O2 (oxygen gas) has 2 parallel lines connecting the atoms, with 2 pairs of dots (known as lone pairs of electrons) on each atom.
Creating Lewis Structures for Larger Covalent Molecules
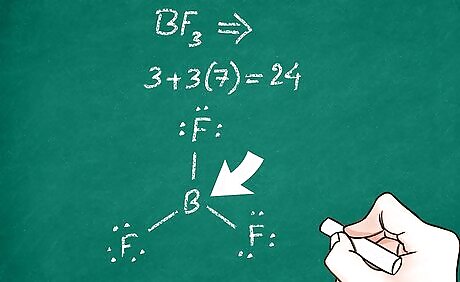
Determine which atom is your central atom. This atom is usually least electronegative. As such, it is most capable of forming bonds with many other atoms. The term ‘central atom’ is used because all the other atoms in the molecule are bonded to this particular atom (but not necessarily to each other). Atoms like phosphorus and carbon are often central atoms. In some more complex molecules, you may have multiple central atoms. Note that in the periodic table, electronegativity increases from left to right and decreases from top to bottom.
Consider the valence electrons of the central atom. As a general (but not all-exclusive) rule, atoms like to be surrounded by 8 valence electrons (the octet rule). When the central atoms bonds to the other atoms, the lowest energy configuration is one that will satisfy the octet rule (in most cases). This can help you determine the number of bonds that will be between the central atom and the other atoms because each bond represents 2 electrons. Some large atoms such as phosphorus can break the octet rule. For example, carbon dioxide (CO2) has 2 oxygens covalently double-bonded to the central atom, carbon. This allows the octet rule to be satisfied for all 3 atoms. Phosphorus pentachloride (PCl5) breaks the octet rule by having 5 bonding pairs around the central atom. This molecule has 5 chlorine atoms covalently single-bonded to the central atom, phosphorus. The octet rule is satisfied for each of the 5 chlorine atoms, but it is exceeded for the phosphorus atom.
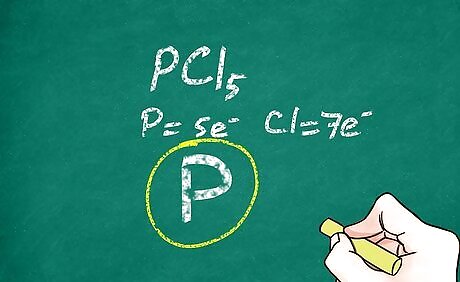
Write the symbol of your central atom. With larger covalent molecules, it is best to start the drawing with the central atom. Resist the urge to write all of the atomic symbols at the same time. Leave plenty of room around the central atom to place your other symbols after you have determined their place.
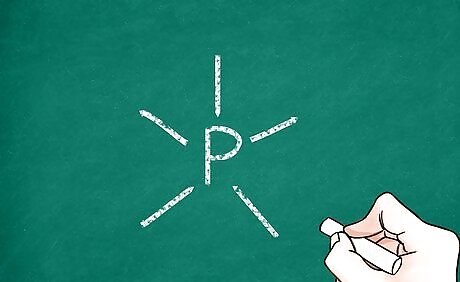
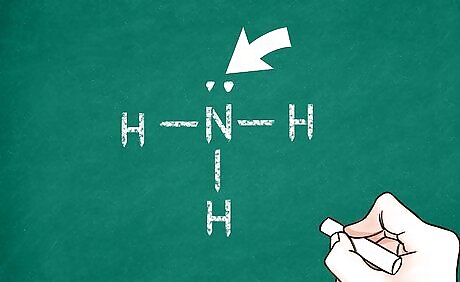
Show the electron geometry of the central atom. For each unshared electron pair, draw 2 small dots right next to each other around the central atom. For each single bond, draw a line going away from the atom. For double and triple bonds, instead of 1 line, draw 2 or 3, respectively. This maps out where the other molecules can bond to the central atom.
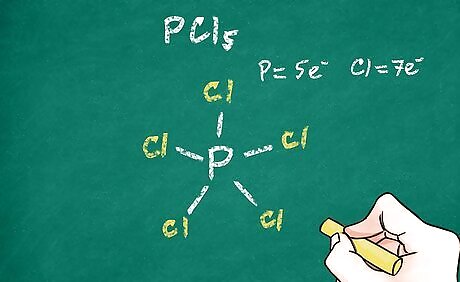
Add remaining atoms. Each remaining atom in the molecule will attach to the one of the bonds coming from the central atom. Write the symbol for each of these atoms at the end of 1 of the bonds you placed around the central atom. This indicates that electrons are being shared between that atom and the central atom.
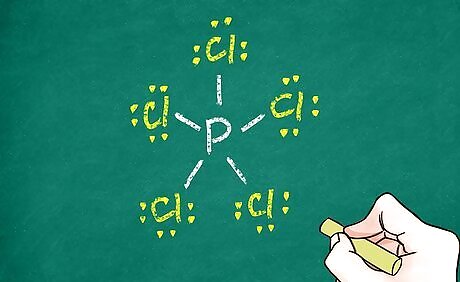
Fill in remaining electrons. Count each bond as 2 electrons (double and triple bonds as 4 and 6 electrons, respectively). Then add electron pairs around each atom until the octet rule is satisfied for that atom. You can check your work on each atom by counting each dot as 1 electron and each bond as 2 electrons. The sum should be 8. Of course, exceptions include atoms that exceed the octet rule and hydrogen, which only has 0 or 2 valence electrons at any given time. When a hydrogen molecule is covalently bonded to another atom, it will have no other unshared electrons surrounding it.
Making Lewis Structures for Ions
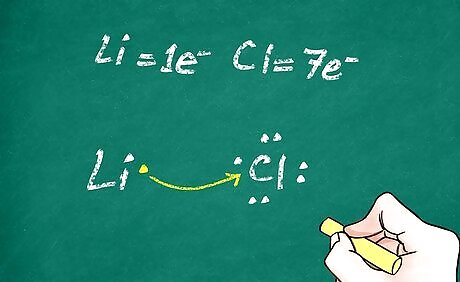
Write the atomic symbol. The atomic symbol for an ion will the be the same as the atomic symbol for the atom that formed it. Leave enough space on the paper around the symbol to be able to add electrons and brackets later. In some cases, ions are polyatomic (more than 1 atom) molecules and are designated by writing the atomic symbol for all atoms in the molecule. To create the symbol for polyatomic ions (such as NO3- or SO42-), follow the instructions for “Creating Lewis Structures for Large Covalent Molecules” in the above method.
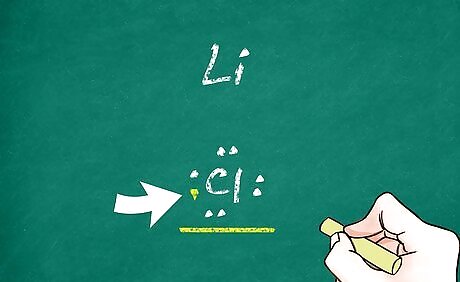
Fill in the electrons. Generally, atoms are neutral and do not carry a positive or negative charge. However, when an atom loses or gains electrons, the balance of positive and negative charge in the atom is altered. Then the atom becomes a charged particle known as an ion. On you Lewis structure, add any extra electrons and remove any electrons that were given up. When drawing the electrons, keep the octet rule in mind. When electrons are lost, a positive ion (known as a cation) is formed. For example, lithium loses its one and only valence electron during ionization. Its Lewis structure would just be ‘Li’ with no dots around it. When electrons are gained, a negative ion (known as an anion) is formed. Chlorine has 7 valence electrons and gains 1 electron during ionization, giving it a full shell of 8 electrons. Its Lewis structure would be ‘Cl’ with 4 pairs of dots around it.
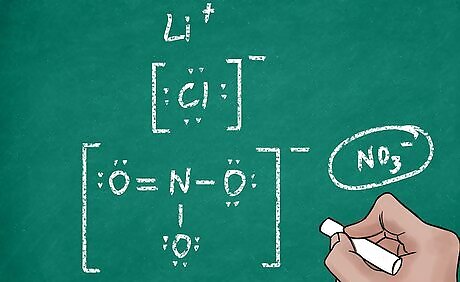
Designate the charge of the ion. Counting dots on every atom would be a tedious way of determining if that atom had a charge. To make the structures easier to read, you need to show that your structure is an ion with some charge. To show this, draw brackets around the atomic (or polyatomic) symbol. Then, write the charge outside the brackets in the upper right corner. For example, the magnesium ion would have an empty outer shell, and would be notated as [Mg].



















Comments
0 comment