views
Basic Molecular Drawing
Learn basic molecular drawing techniques. If already familiar with drawing molecules, proceed to Part 2: "3-Dimensional Structure of Organic Molecules."
Consider the name. Review IUPAC molecule naming at Name a Hydrocarbon Chain Using the IUPAC Method and identify the molecule being studied.
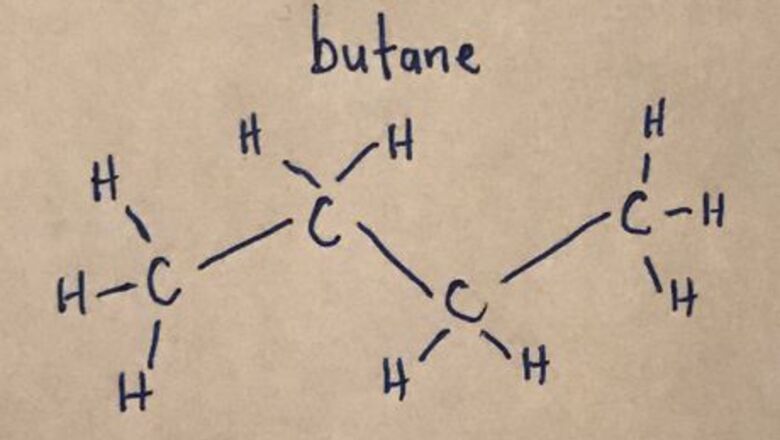
Count the number of carbon atoms. This will be denoted by using the prefix on the name of the chemical, see the list below. 1 carbon: Meth 2 carbons: Eth 3 carbons: Prop 4 carbons: But 5 carbons: Pent 6 carbons: Hex 7 carbons: Hept 8 carbons: Oct 9 carbons: Non 10 carbons: Dec
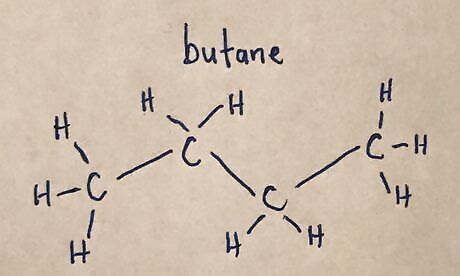
Draw the main carbon chain. Be sure to include hydrogens attached to the carbon atoms.20170321_012938450_iOS (2).jpg
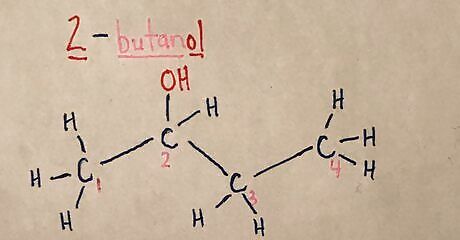
Add any inorganic atoms to the chain. Refer back to the IUPAC name to see where the inorganic groups are located. Examples of inorganic side groups include -OH, -COOH, NH2, Cl, Br, etc.20170321_013413930_iOS (2).jpg
3-Dimensional Structure of Organic Molecules
Learn basics of stereochemistry. If already familiar with stereochemistry, proceed to Part 3: "Drawing a Newman Projection."
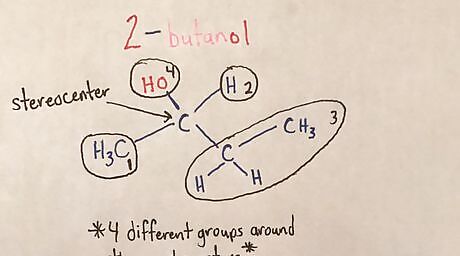
Identify the stereocenter(s). A stereocenter is a carbon atom that has four different groups/atoms attached to it and contains 3-Dimensional characteristics (wedge and dash). A wedged line means the atom is coming "out of the plane" or towards you. A dashed line means the atom is going "into the plane" or away from you.20170321_020112987_iOS (2).jpg
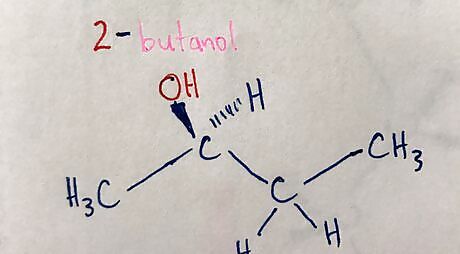
Add in wedges and dashes to stereocenters. Each side group on the stereocenter can be on a wedge or a dash.20170325_025252236_iOS (2).jpg
Drawing a Newman Projection
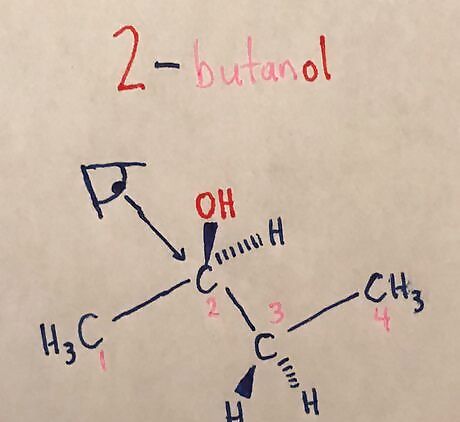
Determine which two carbon atoms are being studied. A Newman Projection focuses on one specific bond of the molecule, and note which atoms are attached to these carbon atoms. Add an eye with an arrow looking down the carbon-carbon bond being studied. Example: 2-3 means looking down the carbon 2 and carbon 3 bond. Notice how the two hydrogens have 3-Dimensional structure. While not a stereocenter, drawing the hydrogens in this way makes drawing the Newman Projection much easier.
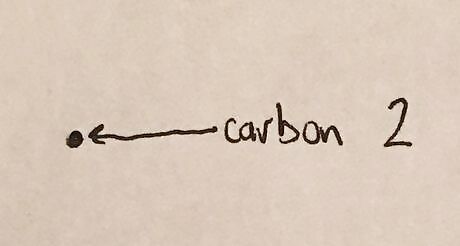
Draw first carbon in the bond as a dot. Note: molecule being drawn in the Newman Projection is 2-butanol.
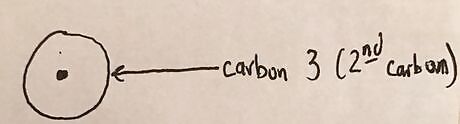
Draw second carbon in the bond as a circle surrounding the dot.
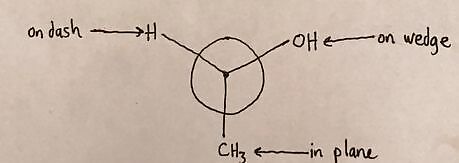
Draw atoms around the first carbon. Look at the 3-Dimensional Drawing from above and label which atoms are attached to this carbon and where each is located. Atoms on a wedge point right Atoms on a dash point left Atoms in the plane of the molecule go up or down.20170321_024406778_iOS (2).jpg
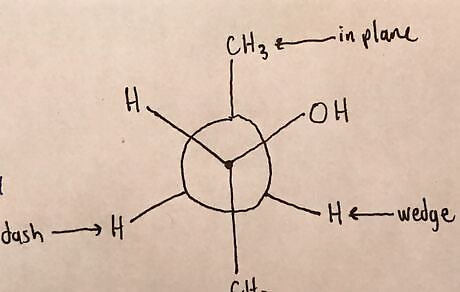
Draw atoms around second carbon. Look at the 3-Dimensional Drawing from above and label which atoms are attached to this carbon and where each is located. Note the 3-Dimensional location of all the atoms from the structure above and check to make sure the Newman Projection matches for each.20170321_024755869_iOS (2).jpg
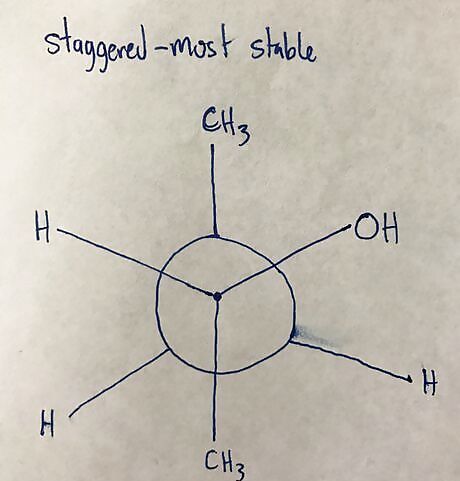
Rotate atoms around second carbon to create new conformation. Each 60 degree turn of the atoms creates a new conformation, and there are six total conformations for each Newman Projection. Staggered is most stable, eclipsed is least stable.20170325_032409022_iOS (2).jpg 20170325_032031093_iOS (2).jpg Completing the other three conformations is done in a similar way, with 60 degree rotations done for each.



















Comments
0 comment